What Are The Best Cannabis Facility Guidelines?
Gold Standard in Facility Development: GPP vs GMP, and what it all means
4trees offers the gold standard in cannabis facility development, but what does that mean? As the cannabis industry continues to grow, cultivators are faced with numerous decisions on how to build their facility or plan for the cultivation practices. One of the most significant decisions in this scenario is whether to use GMP or GPP for their cannabis cultivation production guidelines. Let’s jump into the differences between GMP and GPP, along with the pros and cons of each set of guidelines.
GMP, or Good Manufacturing Practice, is a set of guidelines created by regulatory agencies to ensure that products are consistently produced and controlled according to the quality standards put in place by each specific trade. GMP guidelines apply to the production of pharmaceuticals, food, and other consumer products, including cannabis. GMP requires a rigorous set of procedures, documentation, and quality control measures that must be followed throughout the entire cultivation process.
On the other hand, GPP, or Good Production Practice, is a less stringent set of guidelines developed by the cannabis industry. GPP is less prescriptive than GMP and allows for more flexibility in cultivation practices. The goal of GPP guidelines are simply to ensure that cannabis products are constantly cultivated and produced in a safe and hygienic manner.
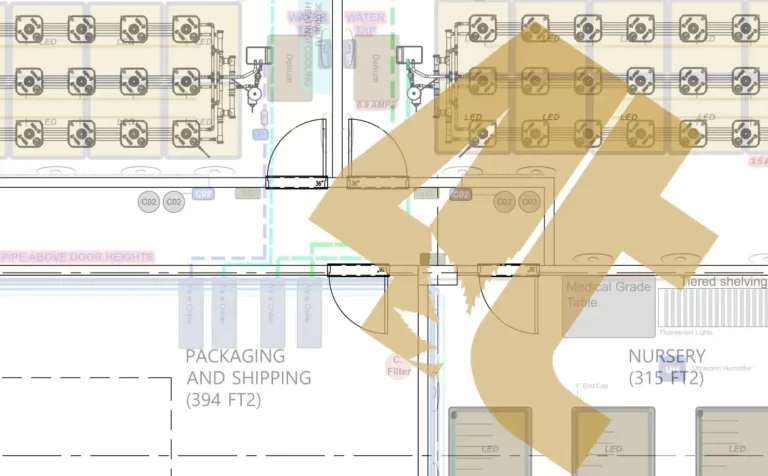
Yes, cannabis cultivation needs strict guidelines too!
While many of you may be moving from a world where black and white poly plastic on the walls was good enough to keep clean, the recreational cannabis world is much different unfortunately, but with good reason. When products are produced for the public most advanced governments around the world where cannabis is legal will have strict guidelines set in place. This is so that the cannabis products being sold are safe for the public, and the staff producing them.
There are several key differences between GMP and GPP. The biggest being the sheer level of documentation required with Good Manufacturing Practices. GMP requires extensive documentation of all processes and procedures, while GPP does not. Additionally, GMP requires more stringent quality control measures, such as batch testing, while GPP allows for more flexibility in testing.
Another significant difference between GMP and GPP is the level of standardization. GMP requires a high level of standardization, while GPP allows for more variation in cultivation practices. This can be advantageous for cultivators who want to experiment with different cultivation techniques and strains.
Cannabis Cultivation Planning Experts
In terms of advantages and disadvantages, GMP is often seen as the gold standard for cannabis cultivation production. Good Manufacturing Practices ensures a high level of consistency, quality, and safety, which can be essential for medical cannabis products. GMP can also be much more costly and time-consuming to implement, however, GPP allows for more flexibility and can be less expensive to implement. It should be noted that the lack of standardization can make it more challenging to produce consistent, high-quality cannabis products, and certain governments will require GMP over GPP guidelines.
In conclusion, the decision to use GMP or GPP for cannabis cultivation production ultimately depends on the cultivator’s goals and resources. While GMP may be the preferred choice for medical cannabis products, GPP may be more suitable for cultivators who want more flexibility and lower costs. No matter what your choice in cannabis cultivation facility design, its important for cultivators to implement clear quality control measures to ensure the safety and consistency of their cannabis products and staff.

